Mechanism of action and rationale for therapeutic effectiveness
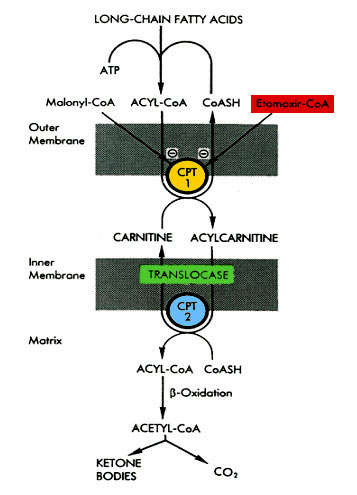
Etomoxir as an ester is saponified in the intestine and after further absoption and transportation similar to any normal fatty acid, Etomoxir is intra-cellular converted to its Coenzyme A ester. In this form Etomoxir acts as a strong inhibitor of mitochondrial CPT 1
(EC 2.3.1.21). This regulatory enzyme is tightly associated with the outer mitochondrial membrane and is responsible for the transport of long-chain fatty acids from the cytosol into the mitochondria for ß-oxidation. The physiological regulator of CPT 1 is malonyl-CoA, a metabolite of fatty acid synthesis (fig. 1).
In this sense, Etomoxir-CoA acts as an analogon of malonyl-CoA, with the difference that Etomoxir-CoA is approximately 100-1000 times more effective in inhibiting CPT 1. Only the (R)-(+)-Etomoxir-CoA, not the (S)-(-)-Etomoxir-CoA, is an effective inhibitor of the enzyme. The nature of inhibition is, in contrast to malonyl-CoA, not reversible but pseudo-reversible, i.e. inhibition is a concentration and time-dependent process, of which the molecular mechanism remains to be clarified. In vivo, loss of enzyme activity is fully reversible, which can be attributed to a re-synthesis of the enzyme (ref. 8).
The inhibitor concentration for 50 % inhibition (IC50) was estimated as 5 to 20 nmol/l, depending on the animal’s state of metabolism – whether or not it has been fed, whether it is diabetic and its state of health. The measured in vitro sensitivity of CPT 1 in rat liver is the same as in rat muscle (IC50: 6–7 nmol/l). The sensitivity of the rat heart CPT 1, however, is lower by a factor of 2. The in vivo sensitivity in rats decreases as follows: liver > heart > muscle. To what extent the different distribution of the substance within the organs or differences in the intracellular activity of Etomoxir-CoA-synthesis is responsible for the shift of the range of inhibition is not yet clear ( ref. 79).
Etomoxir specifically inhibits the regulatory enzyme CPT 1; there is no influence on either CPT 2, the constituent part of membrane, or other ß-oxidation enzymes. This circumstance together with organ specific inhibitability of CPT 1 makes Etomoxir suitable as an effective regulator of the fuel metabolism in cells. All of the observed pharmacodynamic actions of Etomoxir are directly or indirectly a result of the shift from fatty acid oxidation to glucose oxidation. The inhibition of fatty acid oxidation does not only result in a depression of ketogenesis, cholesterol synthesis and gluconeogenesis. Through deinhibition of pyruvate dehydrogenase there is an activation of glucose oxidation in all cells and a feedback inhibition of endogeneous fatty acid synthesis, especially in the liver (ref.82).