
Chemical and pharmaceutical properties
Drug substance
Etomoxir (INN) is chemically ( R )-( + )-2-[6-(4-chlorophenoxy)hexyl]oxirane-2-carboxylic acid ethyl ester.
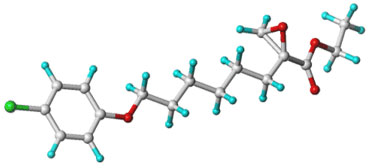

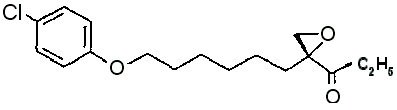
mol. wt: 326,8
Etomoxir is a colourless solid with a melting point of 38°C. Etomoxir is insoluble in water and soluble in organic solvents (alcohol, ether, acetone, dichloromethane, toluene). The sodium salt of Etomoxir is water soluble (about 10 mg/ml).
The structure of Etomoxir is given by its synthesis and is confirmed by spectroscopic data (1H-NMR, IR, UV, MS) and elementary analysis.
The Etomoxir molecule has an asymmetric centre at the C(2) atom of the oxirane ring and is synthesized as the ( R )-( + )-enantiomer. The assignment of the absolute configuration is based on a comparison of the specific rotation (α22D= + 9.9 degree (c=1, CHCl3) with the literature value of enantio-specifically prepared reference substance.
Drug product
Etomoxir is dissolved in Miglyol at 30-35°C and put into soft gelatin capsules. Each capsule contains the active material Etomoxir and Miglyol as filling material. The capsule material contains gelatin, titanium dioxide, glycerol and Anidrisorb 85/70. The procedure and methods employed and specifications used in the process of manufacture or assembly ensure the uniformity of each medicinal product. Evidence of the stability of the medicinal product and its bioavailability for the intended use was given by Byk Gulden Pharmaceuticals GmbH, Constance (Germany) and is applied by HHAC Labor Dr. Heusler, Stutensee (Germany). The test for purity and the assay were carried out by high pressure liquid chromatography (HPLC). The repeated stability tests indicate stability over at least 20 years.
